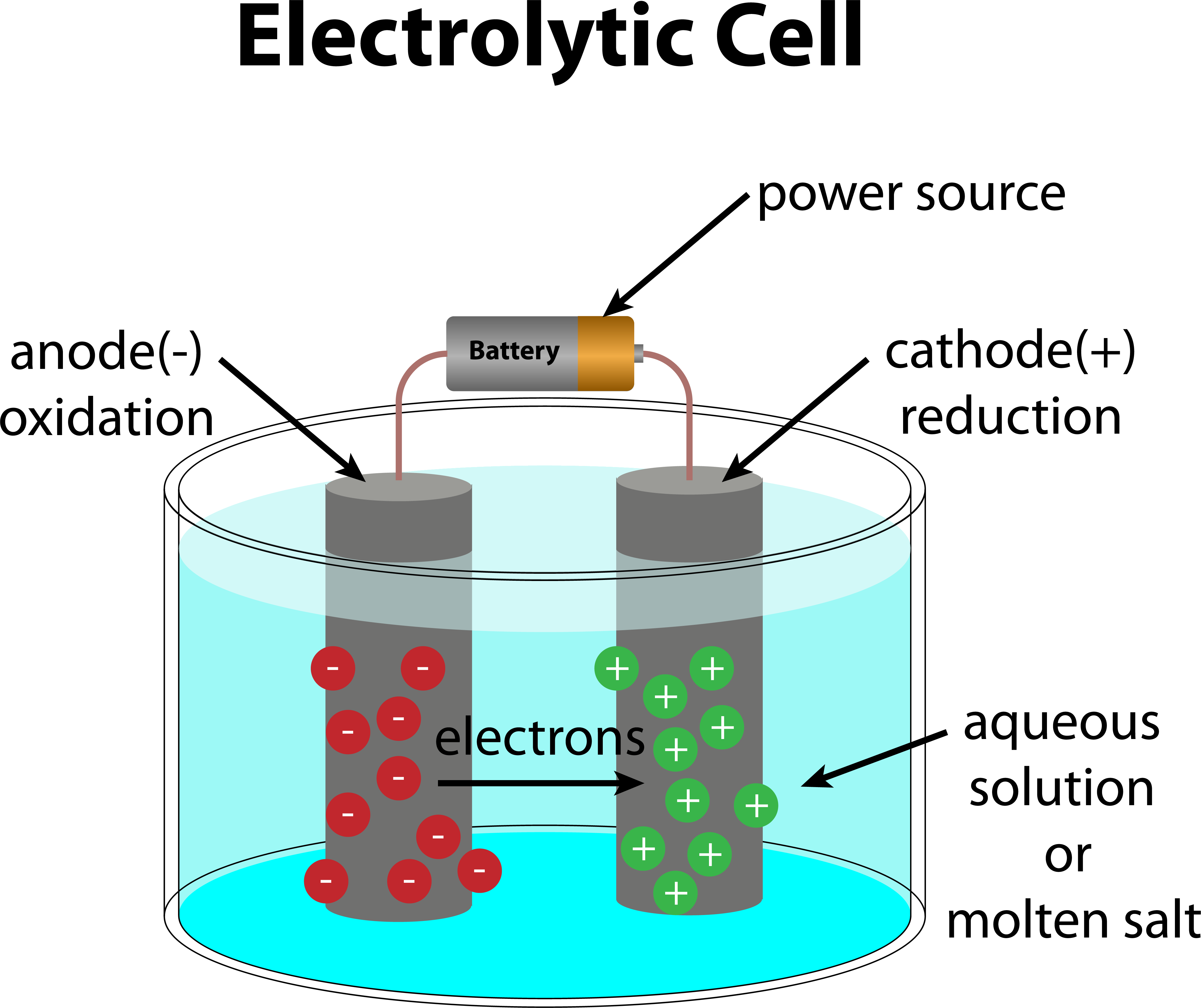
Examples Of Electrochemical Electrolytic Cell . Here, the redox reaction is spontaneous and is. electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. an electrolytic cell converts electrical energy into chemical energy. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. it is also possible to construct a cell that does work on a chemical system by driving an electric current through the system. An electrochemical cell splits the oxidant and reductant in a manner that allows. components of electrochemical cells.
from www.yaclass.in
Here, the redox reaction is spontaneous and is. components of electrochemical cells. electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. An electrochemical cell splits the oxidant and reductant in a manner that allows. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. an electrolytic cell converts electrical energy into chemical energy. it is also possible to construct a cell that does work on a chemical system by driving an electric current through the system.
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science State Board, Class 9.
Examples Of Electrochemical Electrolytic Cell an electrolytic cell converts electrical energy into chemical energy. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell splits the oxidant and reductant in a manner that allows. it is also possible to construct a cell that does work on a chemical system by driving an electric current through the system. components of electrochemical cells. electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Here, the redox reaction is spontaneous and is. an electrolytic cell converts electrical energy into chemical energy.
From chem.libretexts.org
Electrolytic Cells Chemistry LibreTexts Examples Of Electrochemical Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows. components of electrochemical cells. Here, the redox reaction is spontaneous and is. it is also possible to construct a cell that does work on a chemical system by driving an electric current through the system. an electrolytic cell converts electrical energy into chemical energy.. Examples Of Electrochemical Electrolytic Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Examples Of Electrochemical Electrolytic Cell Here, the redox reaction is spontaneous and is. components of electrochemical cells. it is also possible to construct a cell that does work on a chemical system by driving an electric current through the system. an electrolytic cell converts electrical energy into chemical energy. an apparatus that is used to generate electricity from a spontaneous redox. Examples Of Electrochemical Electrolytic Cell.
From printablebispa6t.z21.web.core.windows.net
Electrochemical Cells Gcse Chemistry Examples Of Electrochemical Electrolytic Cell it is also possible to construct a cell that does work on a chemical system by driving an electric current through the system. Here, the redox reaction is spontaneous and is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell splits the oxidant. Examples Of Electrochemical Electrolytic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Chemistry Review Examples Of Electrochemical Electrolytic Cell electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. it is also possible to construct a cell that does work on a chemical system by driving an electric current through the system. components of electrochemical cells. an electrolytic cell converts electrical energy into chemical energy. an apparatus that. Examples Of Electrochemical Electrolytic Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Examples Of Electrochemical Electrolytic Cell components of electrochemical cells. electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. an electrolytic cell converts electrical energy into chemical energy. An electrochemical cell splits the oxidant and reductant in a manner that allows. it is also possible to construct a cell that does work on a chemical. Examples Of Electrochemical Electrolytic Cell.
From www.slideserve.com
PPT CHAPTER 6 ELECTROLYSIS PowerPoint Presentation, free download ID5406391 Examples Of Electrochemical Electrolytic Cell an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. an electrolytic cell converts electrical energy into chemical energy. components of electrochemical cells. it is also possible to construct a cell that does work on a chemical system by driving an electric current through the. Examples Of Electrochemical Electrolytic Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Examples Of Electrochemical Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows. Here, the redox reaction is spontaneous and is. electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. an electrolytic cell converts electrical energy into chemical energy. components of electrochemical cells. it is also possible to construct. Examples Of Electrochemical Electrolytic Cell.
From www.dreamstime.com
Electrolytic Cell Infographic Diagram with Components Stock Vector Illustration of education Examples Of Electrochemical Electrolytic Cell an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. Here, the redox reaction is spontaneous and is. it is also possible to construct a cell that does work on a chemical system by driving an electric current through the system. components of electrochemical cells. . Examples Of Electrochemical Electrolytic Cell.
From in.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram Cell diagram Examples Of Electrochemical Electrolytic Cell an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. An electrochemical cell splits the oxidant and reductant in a manner that allows. an electrolytic cell converts electrical energy into chemical energy. components of electrochemical cells. Here, the redox reaction is spontaneous and is. it. Examples Of Electrochemical Electrolytic Cell.
From www.ck12.org
Electrolytic Cells Example 1 ( Video ) Chemistry CK12 Foundation Examples Of Electrochemical Electrolytic Cell an electrolytic cell converts electrical energy into chemical energy. electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. components of electrochemical cells. An electrochemical cell splits the oxidant. Examples Of Electrochemical Electrolytic Cell.
From studylib.net
Electrochemical cells Examples Of Electrochemical Electrolytic Cell an electrolytic cell converts electrical energy into chemical energy. An electrochemical cell splits the oxidant and reductant in a manner that allows. components of electrochemical cells. electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. an apparatus that is used to generate electricity from a spontaneous redox reaction or,. Examples Of Electrochemical Electrolytic Cell.
From courses.lumenlearning.com
Electrolysis Chemistry Examples Of Electrochemical Electrolytic Cell electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. components of electrochemical cells. An electrochemical cell splits the oxidant and reductant in a manner that allows. it is. Examples Of Electrochemical Electrolytic Cell.
From www.researchgate.net
Example of an electrochemical cell consisting of two electrolytic... Download Scientific Diagram Examples Of Electrochemical Electrolytic Cell electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. components of electrochemical cells. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. it is also possible to construct a cell that does work on a chemical system. Examples Of Electrochemical Electrolytic Cell.
From www.thoughtco.com
Electrochemical Cell Definition Examples Of Electrochemical Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows. components of electrochemical cells. Here, the redox reaction is spontaneous and is. electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. it is also possible to construct a cell that does work on a chemical system by. Examples Of Electrochemical Electrolytic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Chemistry Review Examples Of Electrochemical Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows. electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. it is also possible to construct a cell that does work on a chemical system by driving an electric current through the system. an electrolytic cell converts electrical. Examples Of Electrochemical Electrolytic Cell.
From www.sliderbase.com
Electrochemical Terminology Examples Of Electrochemical Electrolytic Cell an electrolytic cell converts electrical energy into chemical energy. Here, the redox reaction is spontaneous and is. electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. An electrochemical cell splits the oxidant and reductant in a manner that allows. it is also possible to construct a cell that does work. Examples Of Electrochemical Electrolytic Cell.
From chemistry.stackexchange.com
electrochemistry Linking an electrochemical cell to an electrolytic cell Chemistry Stack Examples Of Electrochemical Electrolytic Cell it is also possible to construct a cell that does work on a chemical system by driving an electric current through the system. Here, the redox reaction is spontaneous and is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. components of electrochemical cells. . Examples Of Electrochemical Electrolytic Cell.
From www.slideserve.com
PPT Electrolytic Cell PowerPoint Presentation, free download ID2809508 Examples Of Electrochemical Electrolytic Cell an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. it is also possible to construct a cell that does work on a chemical system by driving an electric current through the system. an electrolytic cell converts electrical energy into chemical energy. electrolytic cell, any. Examples Of Electrochemical Electrolytic Cell.